
Global Collaboration
With the present COVID-19 pandemic, efforts are made to find treatments that are efficient to treat patients afflicted with the symptoms created by the virus, which inter alia affects the respiratory tracts, causes high fever, loss of senses of taste and/or smell, shortness of breath or difficulty breathing, severe acute respiratory syndrome, etc. The reaction of each patient to the virus is individual and varies according to many factors, including age and background illnesses.
It is therefore clear that it is imperative and extremely important to find treatments that may ameliorate the conditions of COVID-19 patients. Based on the available evidence we believe that our mix of API and natural health products may be effective agents in the treatment of COVID-19.
We have now started to conduct clinical trials to provide compositions that are effective in ameliorating the symptoms of the illness caused by the Sars-Cov-2 virus.
Our Team
Headquartered in Israel, we brought together industry’s leading scientists and highly-reputable R&D and production teams to support our research with strong expertise in cutting edge technologies. Our professional and experienced team is focused on finding the right treatment while maintaining high quality and effective results.
Our Progress
Our experts started with a clean slate. Bringing in the best ingredients known from the Pharmaceutical and Natural fields. Working in unisys, several formulas were proposed that have shown high anti-viral properties as well as overall increase in the body’s own immunity system.
Together with and under the care of a medical teams around the country, we tested various strengths and compounds. After several months of hard work in the lab and in the field with Verified COVID-19 patients, we believe a breakthrough formula has been achieved.
Our Results
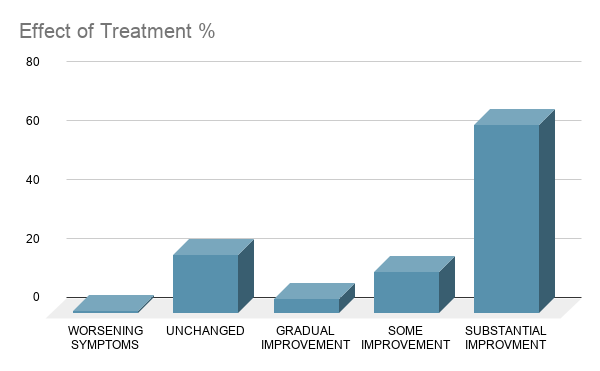
For 65% of patients the treatment showed significant and rapid improvement for reducing the symptoms of COVID-19 and in many cases patients that were previously positive were now negative for SARS-CoV-2, all showing results with an average of 2-7 days.
15% of patients showed improvement with their symptoms.
20% of patients displayed no change in their condition while taking the treatment.
Less than 1% of patients have shown worsening in their symptoms.
The treatment had Zero mortality rate.
Next Step Clinical Trials
Moving forward with our patent pending formula we are looking for Global Partners to work with us in performing Clinical Trials. Our certified lab has created a custom tailored Kit ready to ship anywhere in the world. We are looking forward to getting our treatment approved with various regulatory bodies as soon as possible.
Our Standards
- cGMP according to required regulations such as :U.S FDA , ICH, EMEA. British MHRA Etc.
- GLP according to OECD, European and US regulations
- ISO 13485
- ISO 9001
Exclusive Global Distributor
Contact Us
Respiztal
Ness Ziona, Israel
info@respiztal.com
For more information about Sars-Cov-2 and COVID-19 please refer to:
Disclaimer:
Respiztal is a treatment formulated to relief the symptoms of COVID-19, a disease caused by the Sars-Cov-2 Virus. The treatment is currently under review and is not making any claim for the effectiveness of its treatment. The statements have not been evaluated by any regulatory body.
